GENERAL QUESTIONS (for all gene therapy learners) and EXPERT QUESTIONS (for those with a more advanced knowledge of gene therapy, including healthcare professionals).
General questions
There are two main types of gene therapy, which differ based on the way they work in the body or in the diseases that they are designed to treat:
- Gene addition therapies focus on treating single-gene diseasesSingle-gene diseasestypes of diseases, also called monogenic diseases, in which a mutation is present in one gene only
See glossary for more terms >, also known as monogenic diseases. Single-gene diseases are appropriate targets for gene addition because they can be treated by inserting functional (or healthy) copies of the gene that is mutated. Some of these diseases include Huntington disease and sickle cell disease.2,3 - Gene editing includes a group of techniques that have the ability to make direct changes in DNA. These techniques are: gene inactivation/disruptionGene inactivationan approach in gene therapy that turns off or reduces the function of a gene in order to have a therapeutic effect
See glossary for more terms > (also called silencing, knockdown, or knockout), and gene correctionGene correctiona technique that corrects a faulty gene with functional genetic material with the aim of correcting the faulty gene
See glossary for more terms >/insertionGene insertiona technique that applies genetic material in order to treat a disease at the genetic level
See glossary for more terms >.4 Because gene editing therapy is still so new, it’s still in clinical trials exploring its possibilities in a range of diseases from genetic diseases like cystic fibrosis or Huntington's disease to blood disorders like sickle cell disease.4
Explore Genehome
Explore Genehome
If you receive gene therapy to treat a disease you are living with, you may still pass the disease on to your children. This is due to the types of cells that are treated with gene therapy. There are 2 primary types of cells in your body: somatic cells and germline cells.3
Germline cells, also called reproductive cells, are sperm and egg cells. These cells are unique because they carry genetic information that is passed down to future generations. Somatic cells are all of the other different cells that make up most of your body, such as skin cells and heart cells.3,5
Current gene therapy techniques aim to correct the genetic mutation responsible for causing a disease in 2 ways: by delivering new, functioning genetic material to specific cells in a patient’s body (gene addition) or by directly editing the mutated gene or a gene that compensates for the function of the mutated gene (gene editing).2,4 Both of these approaches are being studied in specific somatic cells that are related to the disease. For example, a gene therapy that seeks to treat a motor neuron disease will target specific neuronal cells that have the disease-causing mutation. Though the mutation is corrected in these specific cells, the individual will still have germline cells in the body that contain the altered gene and pass it on to their children.3
Though gene therapy in germline cells is a possibility, there are many important and serious ethical questions that are raised due to its effect on future generations and unknown, long-term side effects. Due to these concerns, human genome-editing is currently prohibited by the Food and Drug Administration (FDA) and the guidelines of the National Institutes of Health (NIH).6
From the initial design and research on gene therapies to administering treatment and follow up, teams of researchers, clinicians, and regulatory bodies work to assess and monitor/study the safety of gene therapy. That said, this is a constantly evolving field. As the industry collectively continues to learn more about the effects and overall safety of gene therapy, they will apply those learnings to these treatments, seeking to improve its safety.
The safety and effectiveness of any gene therapy is monitored carefully in the period immediately after treatment. Patients who receive gene therapy in clinical trials will be asked to follow-up with their treatment center and physician for a number of years to track any side effects that may develop and the effectiveness of treatment over time. Patients may also be enrolled in registries to help track the long-term outcomes of treatment.
Much like how each person’s genes are unique to them, the gene therapy treatment process is unique to every patient. Depending on a person’s specific disease and treatment goals discussed with their care team, the treatment process will look different from person to person.
However, there are 4 main steps that gene therapy generally follows:
- Preparation
- Consultation
- Treatment
- Recovery/follow-up
Explore Genehome
Explore Genehome
Patients and physicians typically work together to determine if gene therapy is an appropriate treatment option. Physicians will have discussions with their patients about their disease as well as the risks and benefits of gene therapy. From there, a plan for gene therapy could be proposed, or a physician will refer their patient to a specialist who will carry on the discussion about the possibility of receiving gene therapy. As gene therapy is still evolving, it's possible a disease state specialist could refer a patient to an expert in gene therapy who may be able to better assess the risks and benefits of the treatment.
To find out if there are gene therapies approved for your condition or if gene therapy clinical trials are enrolling, start by asking your physician. You can also check with a disease-specific patient advocacy organization or visit clinicaltrials.gov to search for any gene therapy trials that are currently being conducted for your disease.
Whether or not conditioning with chemotherapy is a part of the gene therapy process depends on the specific gene therapy you receive.
If chemotherapy is a part of treatment, you will want to discuss with your doctor and your care team the kinds of side effects you might experience. You may also want to find out how they will help to manage those side effects. If there is the possibility that chemotherapy will affect your fertility (your ability to produce children), you should discuss with your doctor your options for fertility preservation prior to gene therapy.
Though gene therapy has a long history of research and development, there has been a lot of interest and discussion around this therapeutic field in recent years.
Some noteworthy events include the Human Genome Sequencing Project that helped determine the sequence of all the human genes and established the location of diseases caused by genetic mutations. There's also been the Nobel Prize in Chemistry 2020 awarded to the creation of CRISPR. Additionally, new gene therapy techniques are still being discovered and published for the medical community to review and discuss.
Explore Genehome
Explore Genehome
Gene therapies are made through a highly-regulated manufacturing process to help determine the quality of the final gene product (also called the drug product). Depending on the type of gene therapy, the steps involved in the manufacturing process will differ. Synthesizing nucleic acid components of CRISPR-Cas9 is needed for gene editing. Viral vectors need to be engineered to deliver specific, functional genetic material into host cells. And genetically modifying a patient's own cells is required when engaging in ex vivo gene therapy.7
Despite all the different ways gene therapies are made, they share a common goal. Because of their complexity, all gene therapies need to have a manufacturing process that is both consistent and able to be reproduced.7
When manufacturing gene therapies, the FDA requires strict adherence to current Good Manufacturing Processes (cGMP) as described in their regulatory guidance documents. The FDA is also responsible for oversight of all manufacturing processes from the very start of developing a gene therapy through the clinical trial development and approval process. These include specific controls that are put into place in every step of creating a gene therapy to help evaluate its safety, purity, and potency.8
As scientists and researchers continue to move forward in their understanding of gene therapy clinical development, they make updates and refinements to manufacturing processes, which continue to be evaluated by regulatory bodies.
While there are costs involved in every step of the gene therapy manufacturing process, here are several reasons that can explain why a gene therapy can involve a high financial cost:
- Gene therapy may be personalized medicine that uses the patient’s own cells, which means it is made specifically for a person and that person only; this potentially carries a higher cost than drugs and treatments that are not personalized and are made with more common starting materials.1
- The technologies used to manufacture gene therapies are complex and specific, and require intensive and thorough quality control measures to help ensure treatments are made correctly.1
- Gene therapy has the potential to replace a lifetime of chronic treatments and all of the costs associated with them. For example, if a person needs prescription medications every month in order to manage their condition (along with associated doctor appointments and hospitalizations), gene therapy may be replacing the cost of those medications and visits over decades of a person’s life. Remember that the goal of gene therapy is to treat the source of disease with a one-time treatment that lasts over the long term. This means that re-treatment or additional treatments may not be necessary after gene therapy.1
Genetic diseases affect each person differently, making a treatment unique for each patient. If you are discussing gene therapy treatment or enrolling in a clinical trial for gene therapy with your healthcare provider and care team, and have questions about coverage, reach out to your insurance carrier to discuss details about your specific coverage plan.
Different gene therapies will have different prices, depending on a variety of factors, including the manufacturing costs, treatment costs, and the disease being treated. Gene therapy has the potential to replace a lifetime of chronic treatments and all of the costs associated with them, which may make the one-time cost of a gene therapy higher than the yearly cost of a chronic treatment.
For example, if a person needs prescription medications every month in order to manage their condition (along with associated doctor appointments and hospitalizations), gene therapy may be replacing the cost of those medications and visits over decades of a person’s life. Remember that the goal of gene therapy is to treat the source of disease with a one-time treatment that lasts over the long term. This means that re-treatment or additional treatments may not be necessary after gene therapy.1
There are joint efforts between companies that manufacture gene therapies and healthcare insurers to determine new payment models for gene therapy that reflect the value of these one-time treatments. For example, there are payment models that spread out the cost of gene therapies over time, and other payment approaches that are tied to the effectiveness of treatment and the results that patients may experience.1
The price of gene therapy and the portion that a patient is responsible for will vary by insurer. Companies that develop gene therapies are committed to working with patients, their families, and the community regarding access to the treatment they are approved for. This includes specific programs and resources that may be available for patients to help with the cost of treatment; however, eligibility for these programs may depend on the type of insurance that the patient has and other eligibility requirements.1
If you are discussing gene therapy treatment with your healthcare provider and care team, and have questions about coverage, reach out to your insurance carrier to discuss details about your specific coverage plan. Patient advocacy organizations may also be a resource for exploring financial support and assistance.
Expert questions
A donor blood stem cell transplant, also known as an allogeneic hematopoietic stem cell transplant (allo-HSCT), is a treatment option that uses cells from another person (a donor) who does not have the disease. The donor’s healthy cells have a functional gene that is transplanted into the body of the person with the disease.9
Gene therapy does not use cells from an external donor. Gene therapy targets diseases at the genetic level by delivering functional genetic material into the patient’s body by using a viral vector (gene addition therapy), or by modifying the patient’s DNA directly in their cells (gene editing).6,9
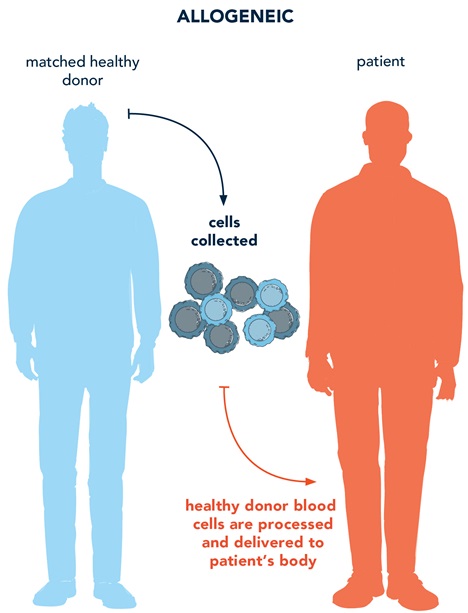
When both gene therapy and allo-HSCT are possible treatment options for a genetic disease, there are several factors to consider for your patient10:
- Availability of donor match allo-HSCT
- Age
- Risk factors (like if allo-HSCT will not cure the disease)
- Comorbidities
- Other risks associated with transplant, including prolonged immunosuppression, infectious complications, and graft failure
While both allo-HSCT (including bone marrow transplants) and ex vivo gene therapy carry the risks of chemotherapy for conditioning, gene therapy currently does not have additional immunological risks (graft rejection, GVHD) associated with allo-HSCT.11 For more information about the potential risks related to gene therapy, please click here.
Helping patients and caregivers understand the potential side effects and risks of chemotherapy is crucial to their treatment decision and experience. Gene therapy research is being conducted on less toxic chemotherapeutic options for conditioning; refer to prescribing information for any given gene therapy for guidance on conditioning regimens used in clinical trials.
Discussions about fertility preservation are important to have with patients and caregivers of children who are undergoing chemotherapy as part of the gene therapy treatment process; options may include12:
- Embryo or egg freezing
- Ovarian or testicular tissue cryopreservation
- Sperm banking or testicular sperm extraction

Keep learning with Genehome
References
1. Salzman R, Cook F, Hunt T, et al. Addressing the value of gene therapy and enhancing patient access to transformative treatments. Molecular Therapy. 2018;26(12):2717-2726. 2. National Institutes of Health. Medline Plus. Genetic disorders. Accessed July 1, 2021. https://medlineplus.gov/geneticdisorders.html 3. Genetic Alliance, District of Columbia Department of Health. Understanding Genetics: A District of Columbia Guide for Patients and Health Professionals. Genetic Alliance Monographs and Guides; 2010. 4. Li H, Yang Y, Hong W, Huang M, Wu M, Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduction and Targeted Therapy. 2020;5(1):1-23. 5. National Institute of Health. Somatic Cells. Accessed May 3, 2021. https://www.genome.gov/genetics-glossary/Somatic-Cells 6. National Institutes of Health. Genetics Home Reference. Help me understand genetics. Accessed May 3, 2021. https://ghr.nlm.nih.gov/primer 7. STAT. What is gene therapy research and how are gene therapy agents manufactured? Accessed May 3, 2021. https://www.statnews.com/sponsor/2020/07/14/what-is-gene-therapy-research-and-how-are-gene-therapy-agents-manufactured 8. Giancola R, Bonfini T, Iacone A. Cell therapy: cGMP facilities and manufacturing. Muscles, Ligaments and Tendons Journal. 2012;2(3):243-247. 9. American Society of Gene and Cell Therapy. Gene and cell therapy FAQ’s. Accessed May 3, 2021. https://asgct.org/education/more-resources/gene-and-cell-therapy-faqs 10. Anasetti C. What are the most important donor and recipient factors affecting the outcome of related and unrelated allogeneic transplantation? Best Pract Res Clin Haematol. 2008;21(4):691-697. 11. Bagley J, Iacomini J. Gene therapy progress and prospects: gene therapy in organ transplantation. Gene Therapy. 2003;10:605-611. 12. Oktay K, Harvey B, Partridge A, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994-2001.